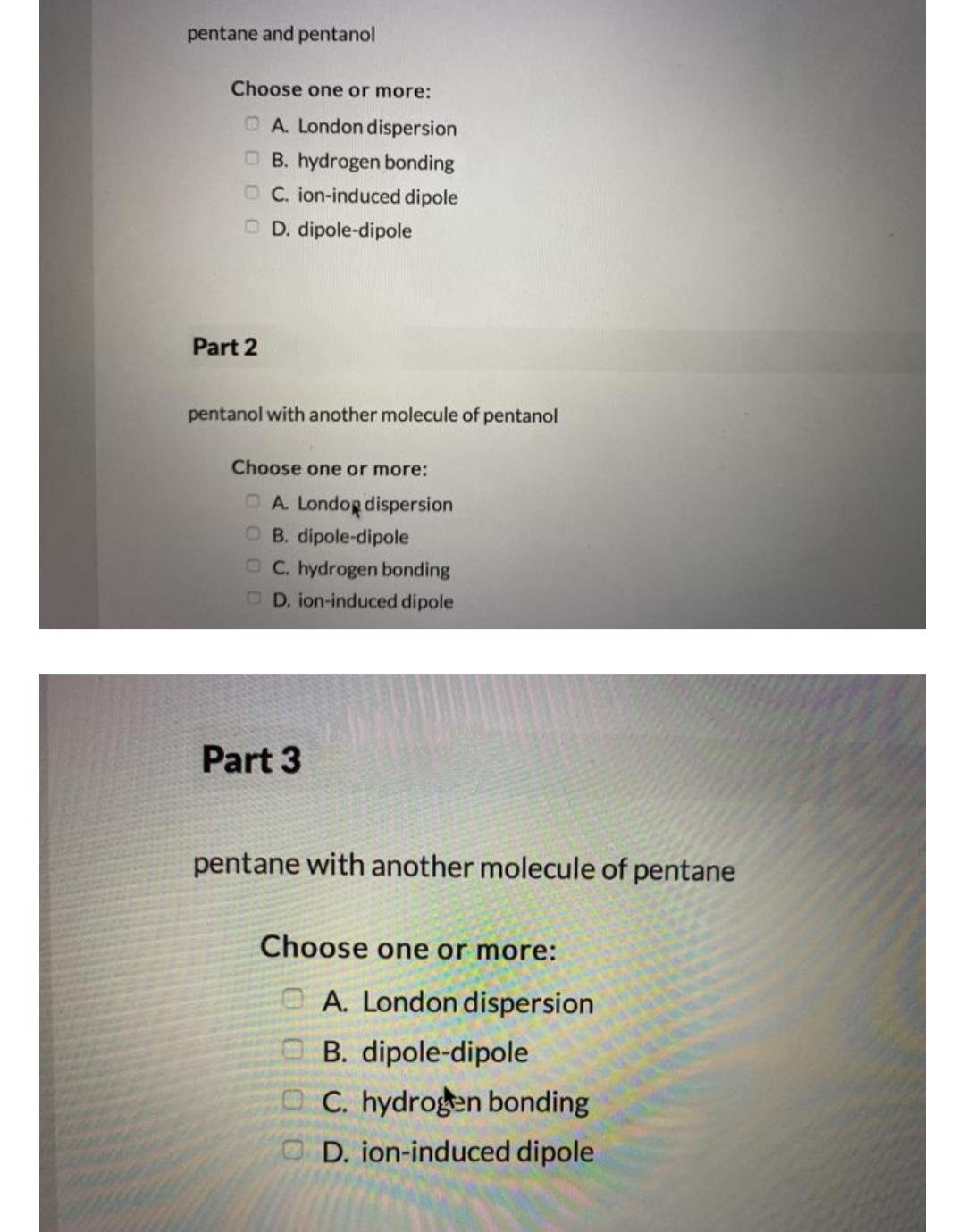
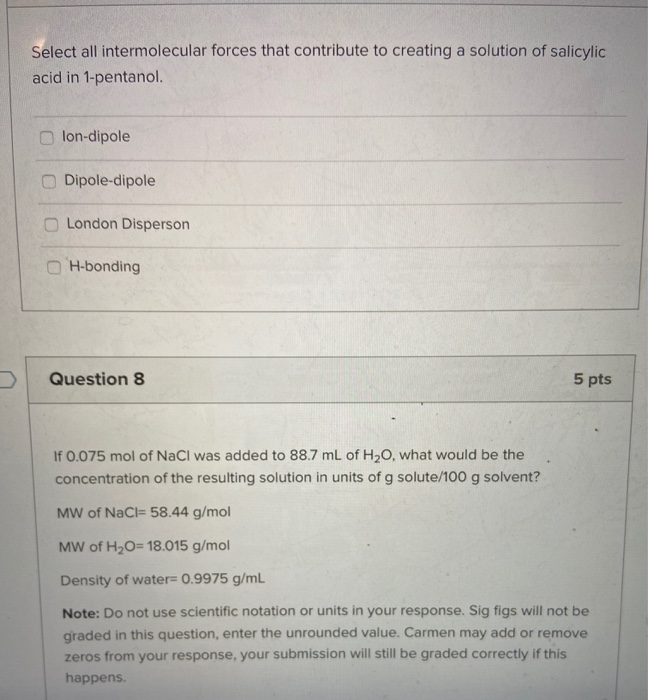
Select All the Intermolecular Forces Associated With 1-pentanol.
E A compound can be separated into different elements with their own unique properties. London Disperson H-bonding Dipole-dipole lon-dipole Select all the intermolecular forces associated with Naci.
Solved Select All The Intermolecular Forces Associated With Chegg Com
Select the compound that should have the lowest boiling point.
. A Compounds have characteristic physical properties. The purpose of the fee is to recover costs associated with the development of data collections included in such sites. There are basically 3 - dipoledipole London Dispersion and H-Bonds.
C CH3CH2MgBr because the non-polar ion interactions are more closely related to dipole-dipole than ionic bonding. It would take more energy to. Follow the links above to find out more about the data in these sites and their terms of usage.
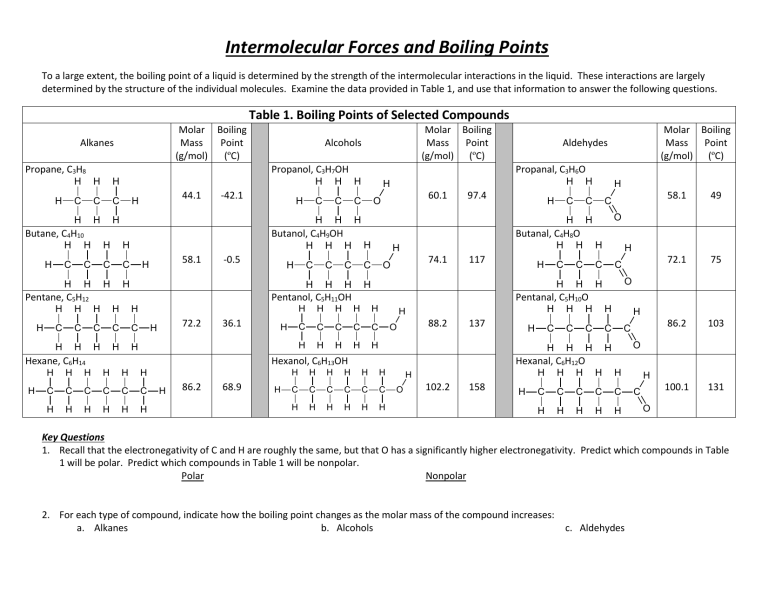
So by looking at the boiling points for a series of molecules the one with the highest value also has the strongest intermolecular forces. 16 2n -- 2416. Chemistry questions and answers.
The snowflake falls yet lays not long Its feathry grasp on Mother Earth Ere Sun returns it to the vapors Whence it came Or to waters tumbling down the rocky slope. CH 3CH 3 CH 3OH and CH 3CH 2OH Answers. Intermolecular Forces and Liquids and Solids In this chapter the concepts ionic and covalent bonding the molecular.
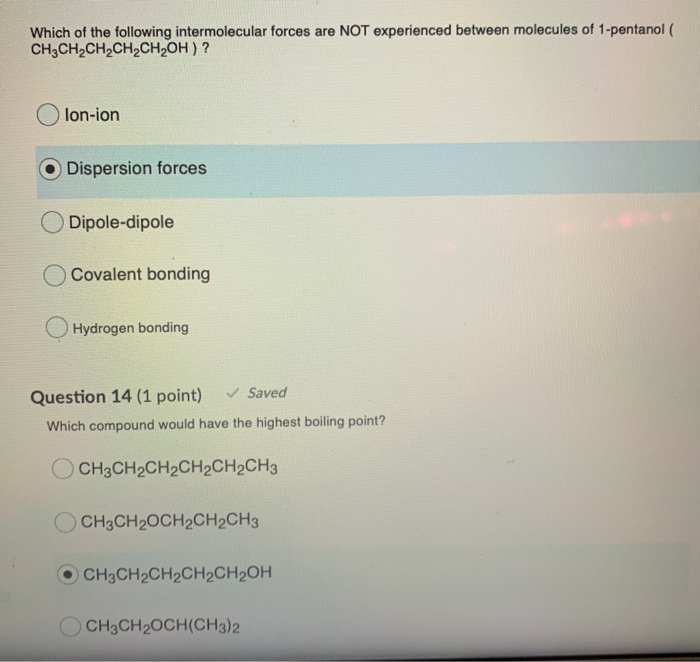
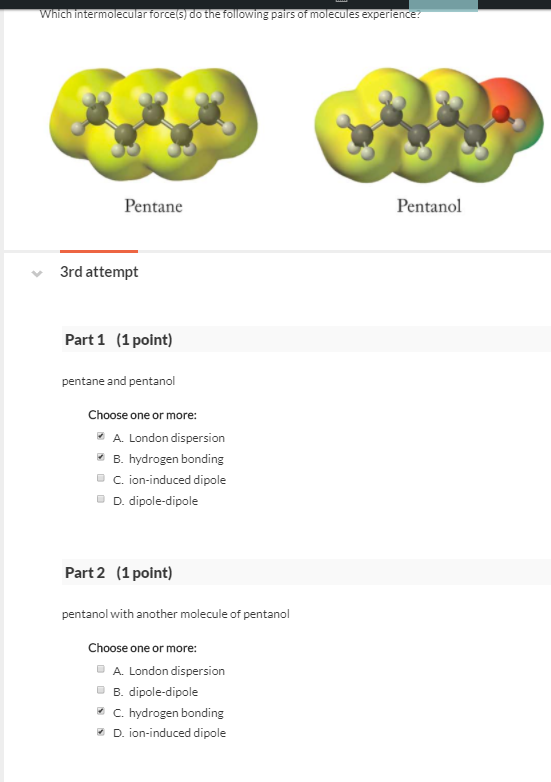
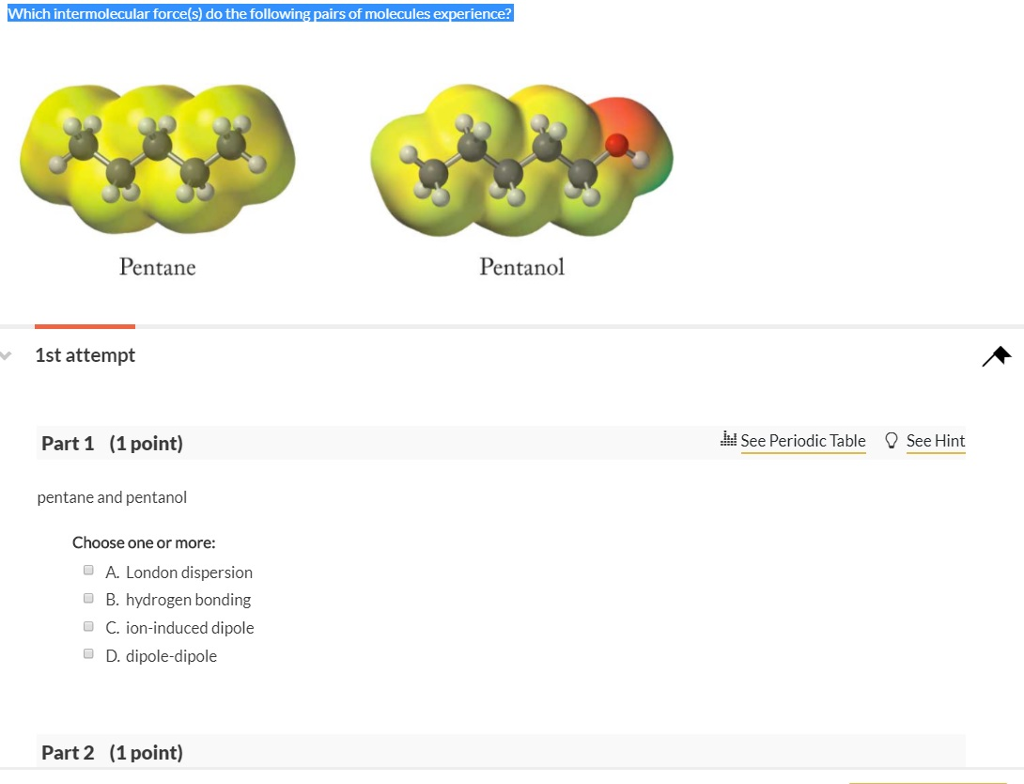
Intermolecular forces 1-pentanol London dispersion forces and H-bonding pentane London dispersion forces 1-Pentanol should have larger intermolecular forces due to H-bonding meaning the molecules are more attracted to each other than in pentane. Compounds with stronger intermolecular forces larger masses and less branching will have higher boiling points. Select the dominant intermolecular force of attraction between C5H12 molecules.
The intermolecular force that is most important in explaining the solubility of alcohols in water is. Impact of Strength of Intermolecular Forces. Identify the intermolecular forces in CH4.
What is the max number of stereoisomers for a molecule with 4 stereo centers. What is the typical mp range of a pure compound. Now 1-propanol has a normal boiling point of 97 98 C.
Select all the intermolecular forces associated with H2O. Up to 10 cash back Boiling point is highly dependent on the intermolecular forces of a compound. Mautner M Intermolecular Forces in Organic Clusters.
Identify the kind of intermolecular forces that would occur between the solute and solvent in Motor oil nonpolar A. Select the ΔH values associated with the dissolution of lithium chloride that are exothermic. The intermolecular forces present in pentanol.
C Compounds are made up of two or more different types of atoms D Compounds can be isolated in pure form. As the carbon chain gets longer the. O A good recrystallization solvent has a structure that is.
Water a small molecule has an exceptionally high boiling point because of intermolecular hydrogen bonding which persists BETWEEN molecules. Intermolecular Forces AIdentify the intermolecular forces present in the following substances and B select the substance with the highest boiling point. What are the features of a good recrystallization solvent.
AHCl because the dipole-dipole interactions of H-Cl better match the intermolecular forces of diethyl ether. The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Select all the intermolecular forces associated with 1-pentanol.
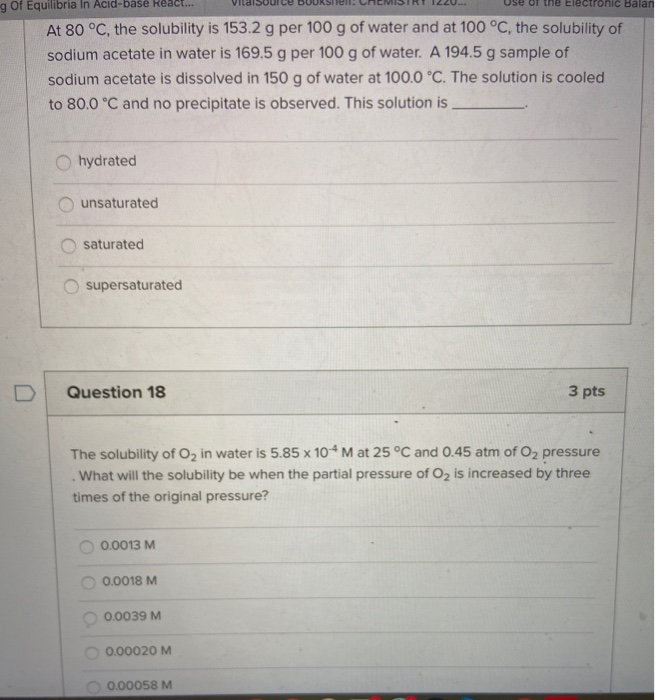
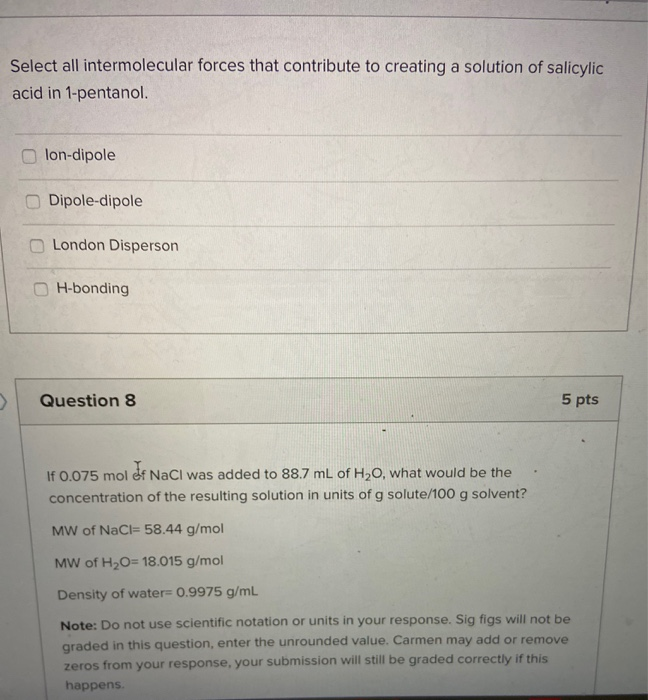
ΔH1 Energy associated with the separation of water. Lon-dipole Dipole-dipole London Disperson H-bonding Question 8 5 pts If 0075 mol of NaCl was added to 887 mL of H20 what would be the. Identify the intermolecular forces in CH3Cl and CO.
O Typically 05 - 15 centigrade. Intermolecular forces 1-pentanol London dispersion forces and H-bonding pentane London dispersion forces 1-Pentanol should have larger intermolecular forces due to H-bonding meaning the molecules are more attracted to each other than in pentane. B H2O because it is capable of hydrogen bonding.
B Compounds have different chemical properties than the elements that compose them. Dipole-dipole interactions are attractive forces among polar molecules. O High molecular symmetry and larger molecular surface area are associated with greater intermolecular forces and higher melting points.
Given these data there is another contributor to intermolecular. The stronger the intermolecular force within a series of like elements the higher the melting and boiling points will be. The length of the alcohol basically determines whether or not they associate with water.
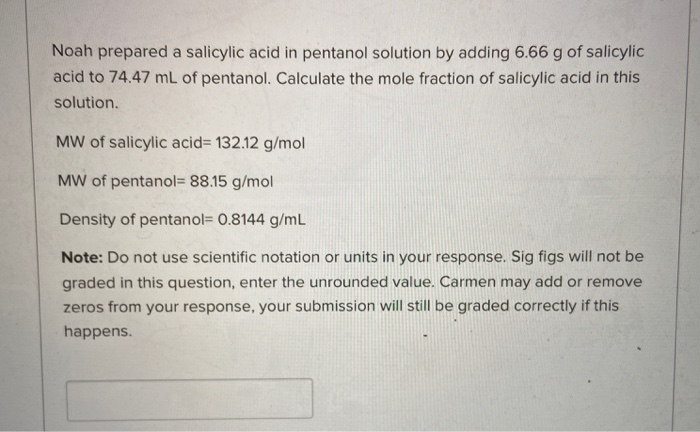
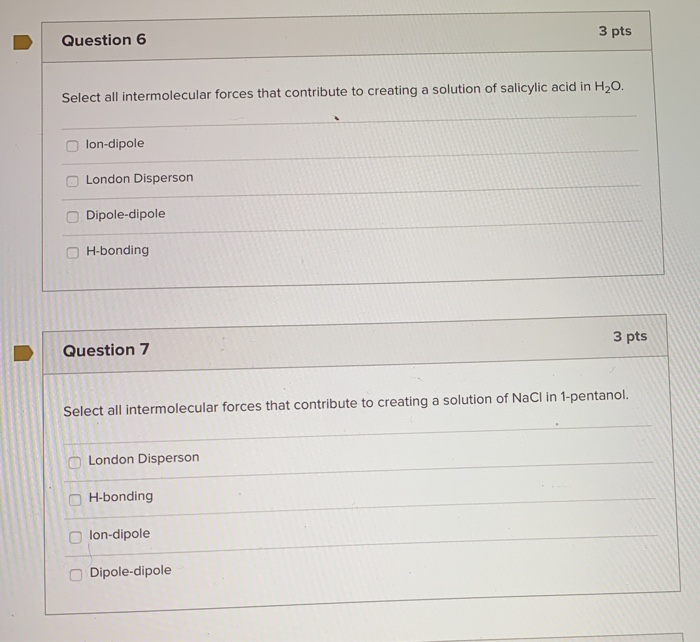
Your institution may already be a subscriber. Den Berg Gerrit JK Heat Capacities and Derived Thermodynamic Functions of 1-Propanol between 10 K and 350 K and of 1-Pentanol between 85 K and 370 K J. London Disperson Dipole-dipole lon-dipole H-bonding Select all intermolecular forces that contribute to creating a solution of salicylic acid in 1-pentanol.
A chemist has three compounds of similar molecular weight but with different dominant intermolecular forces. B CH 3CH 2OH. What type s of intermolecular force is are common to each of the following.
Short chain alcohols have intermolecular forces that are dominated by H-bonds and dipoledipole so they dissolve in water readily infinitely for methanol and ethanol. What type s of intermolecular force is are common to each of the following. Select all the intermolecular forces associated with H2O.
Lon-dipole London Disperson H-bonding Dipole-dipole. Evaporation requires the breaking of all intermolecular forces. Compounds II and III only exhibit intermolecular London dispersion forces so they would be the two lowest boiling compounds weakest intermolecular forces.
A CH 3CH 3 has only dispersion forces whereas the other two substances have both dispersion forces and hydrogen bonds. 1-pentanol b 2-pentanol c 3-pentanol d cyclopentanol B. And we compare this to that of isopropanol 826 C and ethanol 780 C.
Polar molecules have permanent dipoles that are formed due to differences in the electronegativities of the atoms that are associated with a covalent bond. These are hydrogen bonds and London dispersion force. Select all that apply 1 Xe and methanol CH3OH alondon-dispersion forces bdipole-dipole chydrogen bonding i put a and b but it was wrong 2CH3OH and acetonitrile CH3CN.
The intermolecular forces depend on the following interactions.

Solved Question 6 3 Pt Select All Intermolecular Forces That Chegg Com

Solved Select All The Intermolecular Forces Associated With Chegg Com
Solved Which Of The Following Intermolecular Forces Are Not Chegg Com
Intermolecular Forces And Boiling Points
Answered Intermolecular Force Bartleby

Solved Select All The Intermolecular Forces Associated With Chegg Com

Solved State The Type Of Intermolecular Forces Present In Chegg Com

Measuring Surface Tension To Investigate Intermolecular Forces Chemical Education Xchange
Solved Ch Intermolecular Force S Do The Following Pairs Of Chegg Com

Discuss 1 Pentanol 1 Decanol And 1 Butanol In Regards To The Principles Of Intermolecular Bonding Which One Of The Molecules Has Stronger Intermolecular Forces And Why Study Com
Solved 3 Pts Question 6 Select All Intermolecular Forces Chegg Com

Intermolecular Forces And Phase Change

Solved Which Intermolecular Force S Do The Following Pairs Chegg Com
Solved Which Intermolecular Force S Do The Following Pairs Chegg Com
Solved Select All The Intermolecular Forces Associated With Chegg Com

Is 1 Pentanol An Ionic Molecular Nonpolar Or Molecular Polar Compound What Intermolecular Forces Are Present Study Com
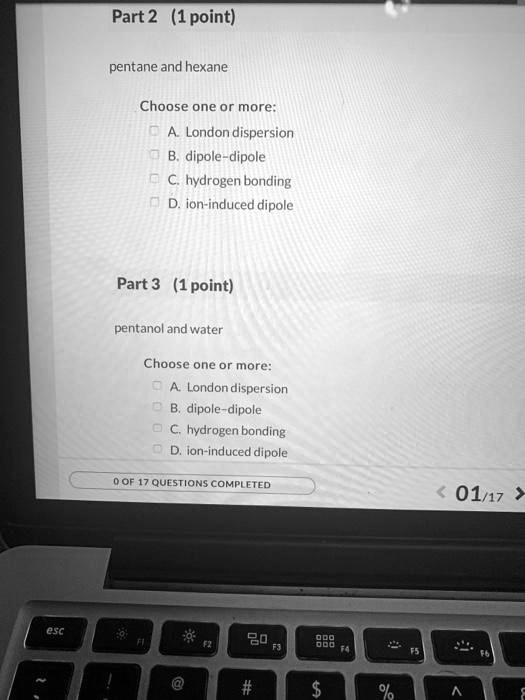
Solved Part 2 1 Point Pentane And Hexane Choose One Or More London Dispersion Dipole Dipole Hydrogen Bonding D Ion Induced Dipole Part 3 1point Pentanol And Water Choose One Or More London Dispersion B
Solved Select All Intermolecular Forces That Contribute To Chegg Com

Solved 2 State The Type Of Intermolecular Forces Present In Chegg Com
Comments
Post a Comment